CTSI's clinical research infrastructure supported the UCLA arm of a multisite study that led to the FDA's recent approval of larotrectinib, a drug that targets specific genetic mutations found in a variety of cancers. Noah Federman, medical director of the CTSI's Clinical and Translational Research Center (CTRC), led the study at UCLA.
Larotrectinib belongs to a class of therapies know as tyrosine kinanse inhibitors (TKI). The second "tissue agnostic" drug approved by the FDA, larotrectinib can be used to treat any kind of cancer that harbors a specific mutation. Most cancer drugs are approved to treat cancers that arise in certain tissues, such as breast, prostate or lung.
The FDA approved use of the drug in cases in which surgery will result in severe morbidity, or the cancer is metastatic, or no alternative treatment exists, or the cancer has progressed following treatment.
Dr. Federman was among the investigators who reported early promising results from the larotrectinib trial in February in the New England Journal of Medicine. More recently, at the Connective Tissue Oncology Society Meeting in Rome, Dr. Federman provided updated results on 120 patients with various types of cancers, including common cancers like lung and colon and rare cancers like soft tissue sarcomas. He reported that cancers in 81 percent of patients responded to the drug at one year. In bone and soft tissue sarcoma, the response rate was an an unprecedented 93 percent.
Dr. Federman, who is also director of the UCLA Jonsson Comprehensive Cancer Center's Pediatric and Soft Tissue Sarcoma Program, conducted his research in the CTRC. CTSI support was invaluable in study activation and successful conduct of the trial, he said.
"We are seeing unprecedented responses in advanced-stage cancer patients. Moreover, the robust efficacy of larotrectinib has been successful regardless of cancer type and age. I never thought I would see these results -- each time I see patients' tumors disappearing in front of my eyes, I am in awe," Dr. Friedman said.
"I can say confidently now that we have changed the paradigm in cancer treatment," he said. "Soon we will eradicate the need for aggressive surgeries and harmful toxins that can put patients at risk in the short and long term."
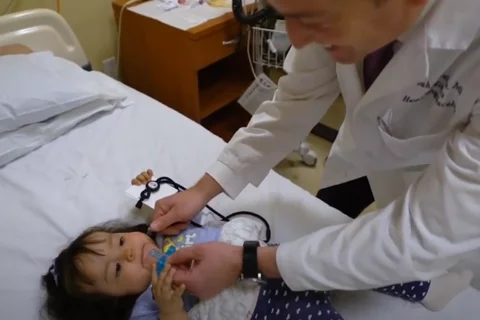
Dr. Federman treated the youngest patient on the trial who was just one month old. "Little Linda" was born with infantile fibrosarcoma, a rare cancer in children that can show up anywhere in the body. There are only 20 cases of the cancer each year in the U.S.
Linda's tumor took up 50 percent of her scalp; Dr. Federman's trial offered her best chance. She became eligible for the trial the day she turned one month old, and she started on treatment immediately. Days later, the tumor began to shrink and today Linda is a rambunctious toddler who loves playing with animals on the family farm.
Further information:
Watch the video for more on the story of little Linda.
Image source: YouTube