Plan for Instruction in the Responsible Conduct of Research
Alert
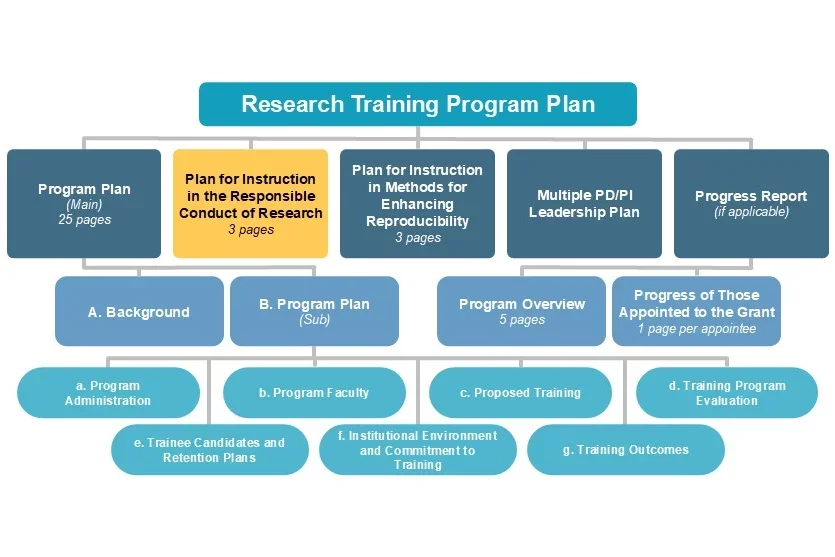
Typically required for training grants, this document should address the following five instructional components and how they will be monitored:
- Format of instruction: In-person, coursework, etc.; solely virtual instruction is not allowable
- Subject matter: Breadth and synergy of subjects covered in the curriculum
- Faculty participation: Roles of faculty and mentors; continued instruction through mentored research projects
- Duration of instruction: Total hours of instruction (at least 8 contact hours)
- Frequency of instruction: Must occur during each career stage and once every four years
Monitoring: Participation in RCR instructions must be monitored
Renewals Only: Describe changes in RCR plan over the previous project period and plans for addressing future weaknesses in current RCR plan. Name all training faculty who have served as course directors, speakers, lecturers, and/or discussion leaders during the previous project period.
Request a Template: GSU has standard boilerplate templates available that can be customized for your project. Contact GSUTraining@mednet.ucla.edu.
Additional Resources:
- NIH Grants Policy Statement on Training in the Responsible Conduct of Research
- Responsible Conduct in Research Training for Bioscientists from UCLA's Bioscience Postdoctoral Affairs
Page Limit: 3 pages maximum
Component Instruction Index
Access detailed instructions for each component of the Research Training Program Plan. If you have further questions contact GSUTraining@mednet.ucla.edu.