Grants Submission Unit
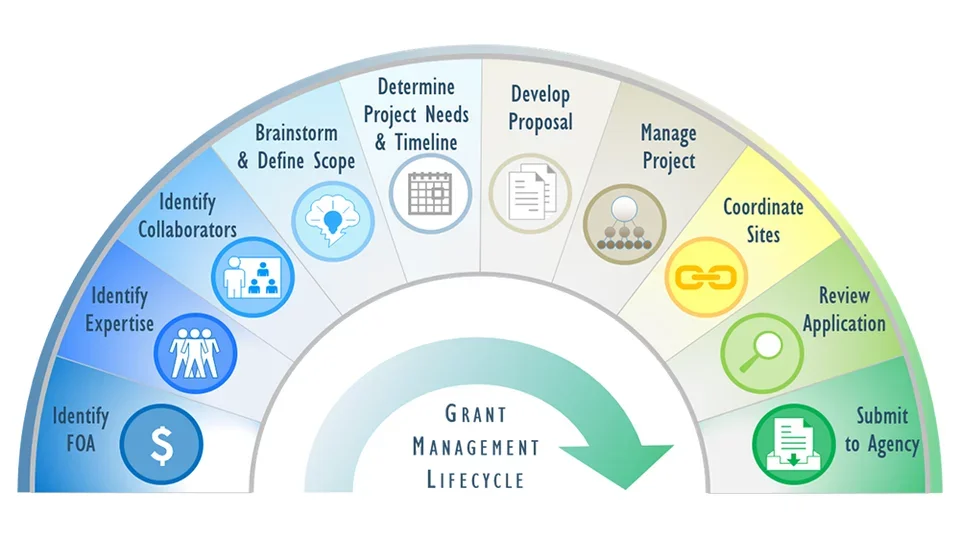
The Grants Submission Unit (GSU) supports the grant application process, with particular emphasis on large and complex grants. Tools and services may include provision of grant writing outlines and detailed checklists of required components, project management and multi-site coordination, review and editing of grant narrative for consistency, clarity and responsiveness to the FOA.
In the cases of large proposals that advance the goals of CTSI, GSU provides hands-on management of the grant-writing process, including collecting biosketches, drafting and soliciting letters of support, and compiling resources and other boilerplate text.
GSU Resources
GSU Services
Editing and Proofreading:
- Drafting grant writing outlines.
- Creating a detailed checklist of required components.
- Reviewing and editing documents to ensure responsiveness to the RFA, clarity and internal consistency across all documents (i.e., ensuring personal statements in biosketches are accurately reflected in the application narrative as well as matched with the budget justification).
Document Management:
GSU can review all required components of the application, organize the collection, distribution and completion of all documentation, as well as draft and distribute required templates. Some of these documents may include:
- Biosketches – Collection as well as review for accuracy and consistency.
- Letters of Support – Individualized for each recipient.
- Cast of Characters – Updated frequently to ensure all key personnel and important administrative staff contacts and their required. documentation are provided.
- Tables and Organizational Charts – Formatting for consistency across the narrative.
- Facilities and Other Resources – Collating, updating and drafting components.
- Boilerplate – CTSI's GSU can provide boilerplate language and examples of program project grant components such as administrative cores and overview sections.
Administrative Support:
With particular emphasis on large complex grants that require excess administrative support, GSU will provide the personnel and expertise needed to pull together all pieces of your grant application. Some of these tasks can include:
- Meeting Initiation and scheduling w/ agenda preparation
- Meeting Minutes and Action Items
- Multisite and sub-award site coordination
- Fielding policy, application, and RFA questions
Brainstorming:
GSU organizes brainstorming sessions with potential collaborators when researchers express interest in funding opportunities. Sessions can be in person or by web/teleconference. CTSI will also provide meeting minutes and action items/deliverables for distribution after each brainstorming session to help keep track of decisions made at each meeting.
Human Subjects and Clinical Trials
The UCLA Clinical and Translational Science Institute (CTSI) Grants Submission Unit (GSU) maintains a number of tools and resources to clarify the requirements for NIH applications regarding human subjects and clinical trial policies. These resources are meant to help guide you through the requirements, based on the type of studies you are proposing within your application.
Visit the NIH Clinical Trials and Human Subjects page to obtain tips on the following components:
- Definition of Clinical Trials
- Types of Human Subjects Studies
- CTSI Resources
- Guidelines and Templates Menu
- Contacts for Additional Support
- Helpful Links from the NIH and Others
- New! Forms-H updates for applications due on or after January 25, 2023.
- FAQs
NIH and NSF Requirements
Visit the UCLA CTSI NIH Requirements page and the NSF Requirements page to obtain tips on the following components related to NIH and NSF applications:
- Authentication of Key Biological and/or Chemical Resources
- Biosketches
- Cover Letters
- Facilities & Other Resources
- Human Subjects
- Letters of Intent
- Letters of Support
- Multi PI/PD Leadership Plan
- Vertebrate Animals
Other CTSI Resources
Regulatory Issues & Grant-Writing Tips
Visit the UCLA CTSI NIH Requirements page to obtain tips on the following regulatory issues and public access requirements:
- Clinical Trials
- Rigor & Transparency
- NIH Public Access Requirements
Grant-Writing Tips
Obtain grant-writing tips on the following:
- General Grant-Writing Tips
- Guide to CTSI Grant Writing Resources
- K and K-to-R Grant-Writing Tips
- NIH Application Guide
- NIH Resources and Grant-Writing Tips
- Search Tools for Funding Opportunities
- UCLA's Commonly Needed Information
- UCLA Office of the Vice Chancellor for Research: Research Enhancement materials
ResearchGo
ResearchGO is CTSI's clinical research portal to resources, expertise and best practices for investigators, study staff, and partners/affiliates.
Manuscript Services (Graphics & Editorial Support)
CTSI offers manuscript services through the Grants Submission Unit (GSU). Go to the Manuscript Services page to get full details and start a consultation.
The following support services are available:
Graphics Support
- Format existing graphics
- Create new graphics, focusing on cohesive design and customized formatting
- Create and format tables, illustrations, and/or charts
- Create scientific illustrations for anatomical, procedural, or engineering concepts
- Create logos for laboratories, centers, or initiatives
Editorial Support
- Review language and content of a manuscript
- Provide consistency in layout and attention to detail
- Provide comments that address flow and logic, word count, and ethical considerations
- Format manuscript per target journal or institutional requirements
- Edit references and citations to adhere to publisher guidelines
Upcoming Events and Deadlines

NIH Data Table Workshop Series

CTSI / Iris Cantor Women's Health Center Awards





